Electrochemical Energy Storage
Electrochemical Energy Storage
Electrochemical Energy Storage is the missing link for affordable and abundant energy systems. Lithium ion batteries (LIBs) dominate these markets, and we are working on developing better anode, cathode, and solid electrolyte materials for LIBs and characterizing the chemistry of performance-limiting processes under different conditions. In addition, we study novel battery and supercapacitor chemistries.
Solid Electrolyte Interphase (SEI) Formation on LIB Electrodes
One strength of LIBs is that they store charge at high voltage (~4V). This yields a high energy density but since few electrolytes have such a wide stability window the electrolyte undergoes either oxidatio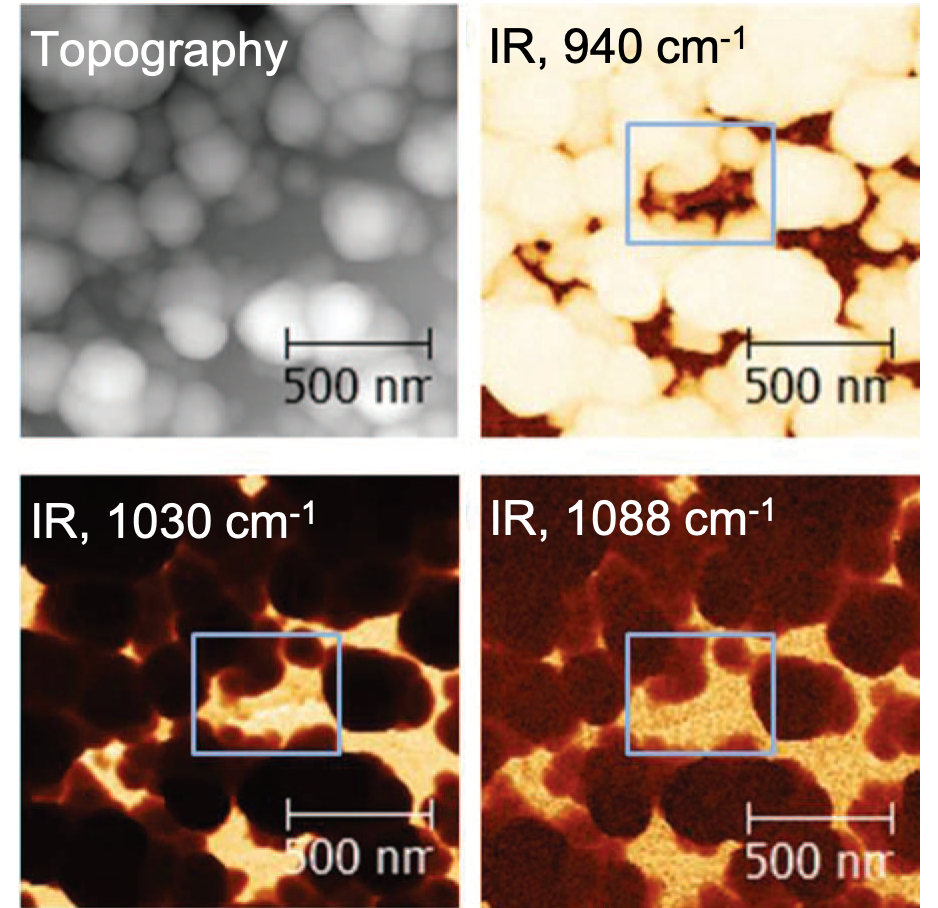 n, or more commonly reduction reactions. Stable performance is achieved if the reaction products build up a stable "solid electrolyte" interphase (SEI) on the respective electrode which is ionically conductive but electronically insulating, kinetically hindering further electrolyte decomposition. Commercial LIBs use carbon-based anodes where stable SEI formation has been accomplished for moderate conditions (temperature, C-rate). Shown right is a near-field infrared map of SEI on graphite, including topographical data and maps at three different wavenumbers corresponding to different SEI species.
n, or more commonly reduction reactions. Stable performance is achieved if the reaction products build up a stable "solid electrolyte" interphase (SEI) on the respective electrode which is ionically conductive but electronically insulating, kinetically hindering further electrolyte decomposition. Commercial LIBs use carbon-based anodes where stable SEI formation has been accomplished for moderate conditions (temperature, C-rate). Shown right is a near-field infrared map of SEI on graphite, including topographical data and maps at three different wavenumbers corresponding to different SEI species.
When anodes are charged at high currents or low temperature, conditions can arise where Li is not just inserted into the anode but plated on the anode. The plated Li can react chemically with existing SEI and/or electrolyte to modify and destabilise the SEI. We are studying the impact of fast charging on SEI composition and stability.
Lithium alloying type high-energy LIB anode materials (e.g., Si, Sn, Ge, Al) are an interesting alternative as they have higher capacity than carbons; however, they exhibit novel SEI formation processes and undergo severe volume changes (~300%) during charge/discharge. SEI formation on such anodes tends to be unstable, and we are investigating their SEI chemistry on alloying type anodes using a number of spectroscopic techniques. In addition, the large volume change applies excess cyclic strain to the SEI layer that forms on the electrode surfaces. We are investigating the quantitative influence of the strain applied to the SEI on the cycling performance of advanced LIB anode materials. Furthermore, we seek to extend our study to develop electrolyte compositions, additives, or artificial surface coatings to address the volume-change induced mechanical instabilities at the electrode-electrolyte interface.
Kinetics of Li storage processes at LIB Electrodes
As a LIB is charged and discharged Li is removed from one electrode and inserted into or plated onto the other electrode. Stable cycling requires that these processes be stable, reversible and repeatable, and the desire to charge portable electronics and electric vehicles rapidly means that these traits need to be maintained at higher and higher current densities. We therefore need to understand and improve the kinetics of these processes.
To this end, we are studying the Li intercalation and plating kinetics on graphite anodes at high currents. Graphite is a highly anisotropic material consisting of stacked sheets of carbon atoms, and graphite particles consequently have basal and edge type surfaces which exhibit different properties. Furthermore, graphite undergoes several phase transitions (stages) as it is lithiated. We are researching Li kinetics on graphite as a function of surface, ageing, and staging in order to find the best way to charge graphitic anodes quickly.
In addition we are studying the kinetics of lithium plating and stripping on Li metal. Li metal is a promising anode material for LIBs due to its high theoretical capacity (3860 mAh/g) and low voltage. However, an SEI layer forms spontaneously on Li in typical electrolytes, and evolves continuously, impacting the impedance for Li+ transport (and the corresponding charge transfer) and the homogeneity of electrodeposition (i.e., formation of dendrites and mossy lithium). Our objective is to develop and apply in situ and ex situ far- and near-field optical multi-functional probes and synchrotron-based advanced X-ray techniques to obtain detailed insight into the active material structure and physico-chemical phenomena at lithium/electrolyte interfaces at a spatial resolution corresponding to the size of basic chemical or structural building blocks, in order to learn how to guide the kinetics towards dense and planar Li plating and stripping.
Novel Materials for LIBs
Commercial LIBs chiefly utilise carbon-based negative electrodes (~372 mAh/g) and layered LiMO2 (M=Co, Ni, Mn) positive electrodes (150-200 mAh/g). Stable performance is achieved but the desire for longer battery life in electronics and more range in electric vehicles requires materials with higher energy densities.
Metallic Glass Anodes
Metallic glasses composed of lithium-alloying elements and lithium-inactive elements are attractive next-generation LIB anode materials. They are amorphous metallic alloys, and can be synthesized with compositions that are normally unstable using far-from-equilibrium synthesis methods. Their unique structure has the potential to resolve many of the issues plaguing lithium-alloying electrodes (e.g., Si, Sn, Ge, Al): the composition can be tuned to stabilize the SEI, inactive components together with the amorphous structure can alleviate strain on lithiation, and high fracture toughness combined with reduced strain can prevent cracking.
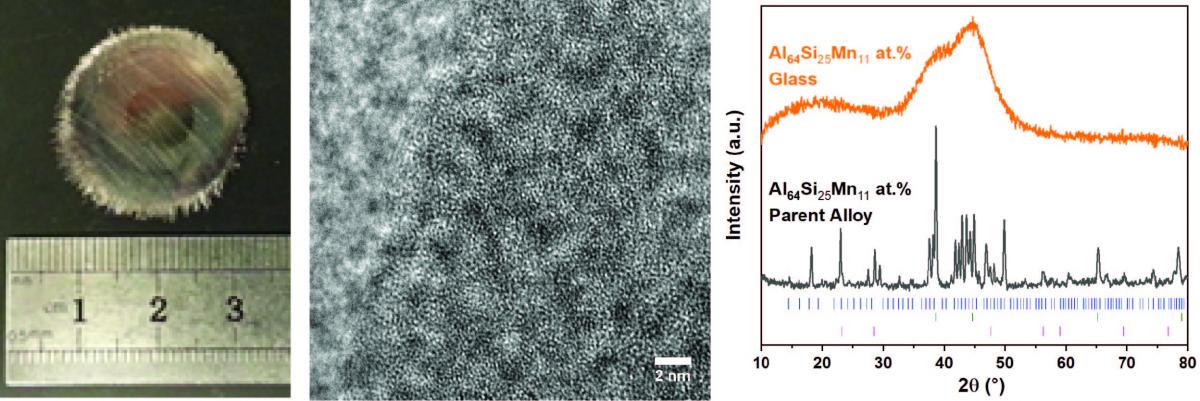
In our group, we successfully synthesized practical scale metallic glasses using a rapid cooling technique. Shown below are a photograph, TEM image, and XRD data for an Al64-Si25-Mn11 metallic glass. The fabricated materials show superior interfacial stability with a reasonable capacity. We are exploring this composition space and conducting further electrochemical/chemical analyses to understand the fundamental mechanism of stable surface passivation.
Disordered Rock Salt Cathodes
Disordered rock salt cathodes are a new class of materials which exploit the percolation theory to generate high energy density cathode materials. These cathode materials display capacities >280 mAh/g, but suffer from low stability over prolonged cycling. This is mainly attributed to an unstable interface due to either densification of the surface or passivation of the surface with high impedance layers combined with transition metal dissolution into the electrolyte.
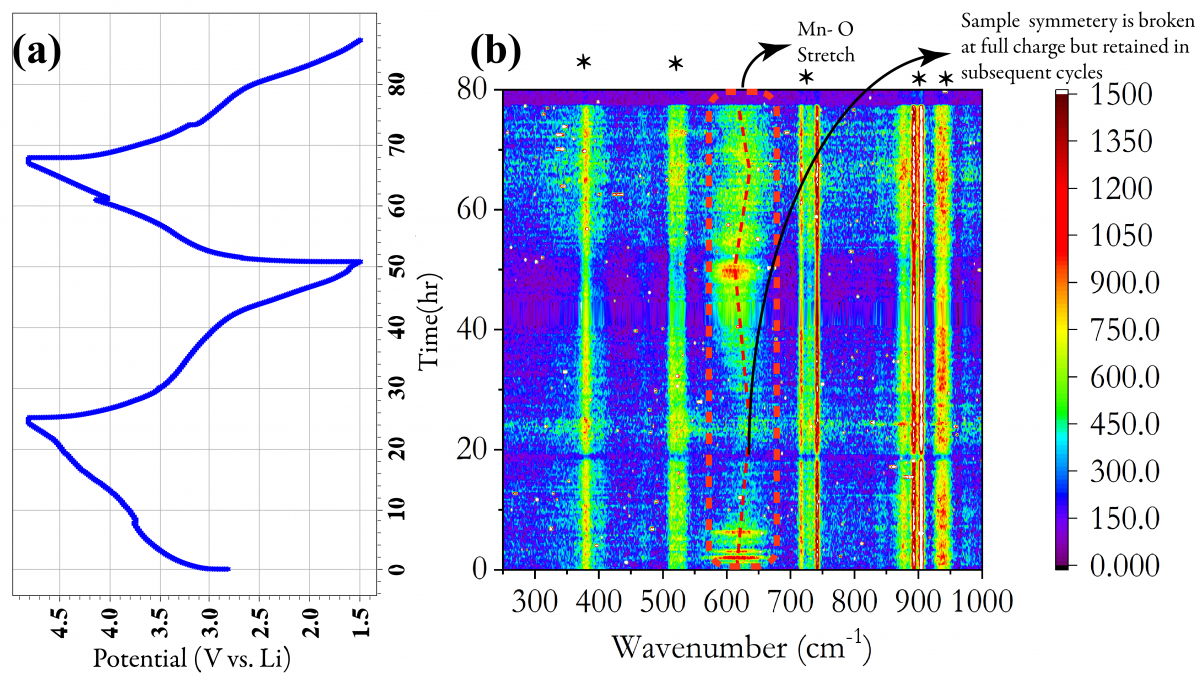
Our focus is using vibrational and X-ray spectroscopic techniques to probe the interface operando and develop a clear understanding of changes occurring in the bulk and the surface. As an example, shown below are operando Raman measurements for Li1.2Mn0.625Nb0.175O1.95F0.05 for the first two cycles. The band at 620 cm-1 is representative of the Mn-O stretch. The break in symmetry on the first charge indicates a distortion of the Mn-O/F octahedra. The second cycle indicates reversible Li intercalation and deintercalation.
Solid-State Electrolytes
Solid-state batteries using a solid electrolyte show potential for providing improved safety as well as higher energy and power density compared to conventional liquid electrolytes. However, the development of solid electrolytes with comparable ionic conductivities and the creation of stable interfaces between solid state electrolyte and electrode components, including the active material, solid electrolyte and conductive additives are the main issues that limit the application of solid state batteries. Our studies mainly focus on the investigation of the diffusion mechanism for lithium ions in solid state electrolytes, and chemical and electrochemical reactions occurring at electrode/electrolyte interfaces during storage and cycling.