Water Desalination
Water Desalination
Global water demand is projected to increase by 55% over the next three decades owing to population growth, industrialization and climate change. Water desalination can help meet this demand provided it is energy efficient. We are developing new technologies that leverage solar energy and renewable electricity to reduce the energy intensity and carbon footprint of desalination technologies, and that expand their reach to alternative water sources.
Thermoresponsive Ionic Liquids and Hydrogels
for Low-Energy Desalination
 The increased use of desalination to meet water demands and emerging wastewater regulations towards zero liquid discharge (ZLD) warrants the evaluation of different technologies for brine management and disposal. Furthermore, the water-energy nexus necessitates the use of renewable sources for wastewater treatment.
The increased use of desalination to meet water demands and emerging wastewater regulations towards zero liquid discharge (ZLD) warrants the evaluation of different technologies for brine management and disposal. Furthermore, the water-energy nexus necessitates the use of renewable sources for wastewater treatment.
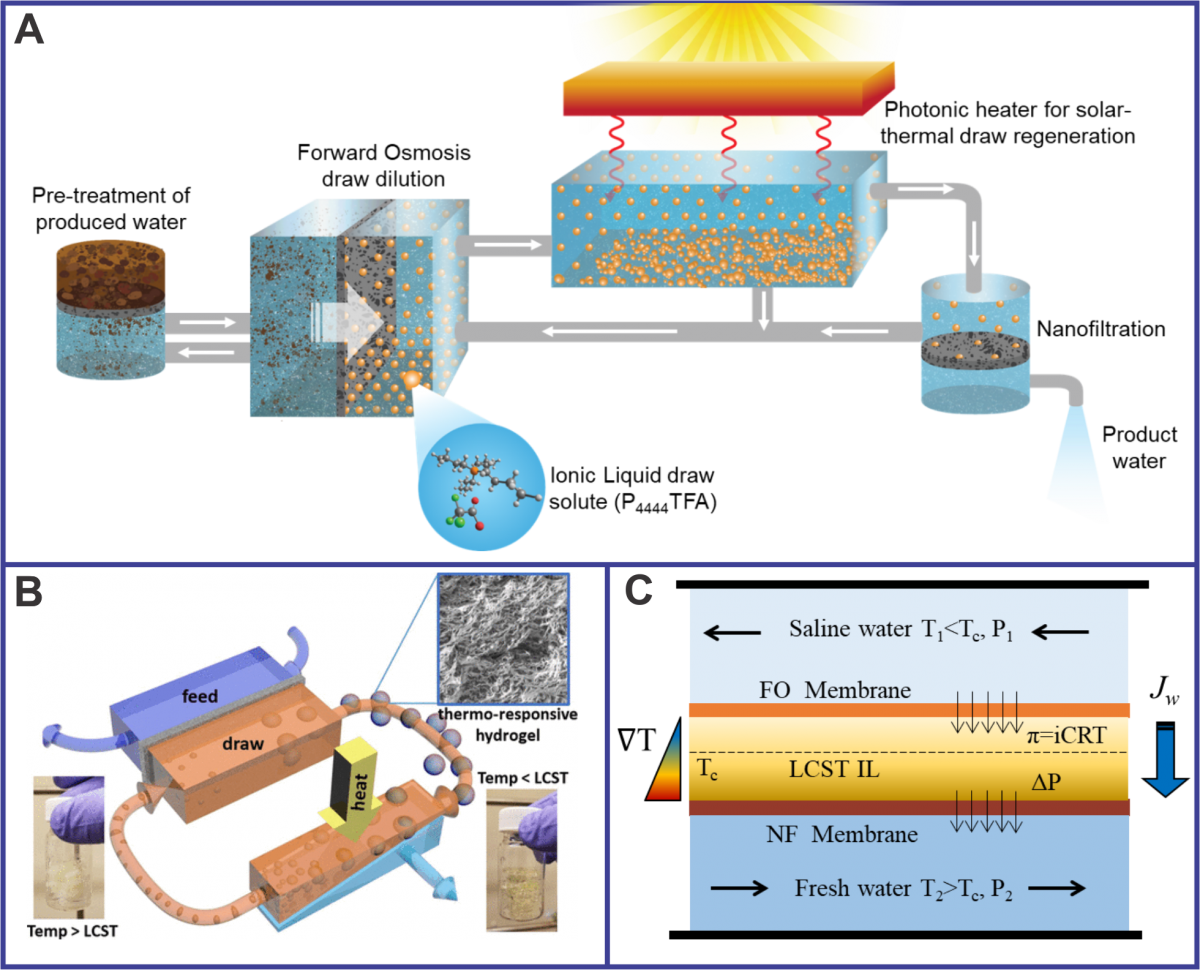
To address this, our research is focused on hybrid membrane-thermal systems that utilize solar energy (A) or waste heat (B) to treat non-traditional waters such as produced water from oil and gas extraction processes with high levels of total dissolved solids that cannot be treated directly by reverse osmosis (RO). We are developing a forward osmosis (FO) system that utilizes thermo-responsive ionic liquids (IL) or IL-polymer hydrogels as draw solutions.
Low-grade solar heat or industrial waste heat is used to drive the separation and regeneration processes to enable an integrated and low cost desalination system. In collaboration with Dr. Ravi Prasher’s group, we are developing photonic heaters to efficiently and reliably harness the solar spectrum for solar-driven desalination. In collaboration with Dr. Jeffrey Urban’s group, we are investigating the molecular-level interactions that enable macroscale phase separation of water and ILs, as well as synthesizing new generations of IL-polymer thermoresponsive hydrogels.
We are also developing hybrid FO-RO based approaches (C) where the FO water permeation and draw regeneration are combined into a single dual-membrane cell. In this continuous desalination process, water is drawn by an osmotic pressure gradient across an FO membrane to form a diluted IL-water solution, which phase-separates into IL-rich and water-rich phases upon heat transfer from the permeate across the RO membrane. Moderate pressure then drives water from the water-rich phase of the separated draw solution through the RO membrane at a much lower pressure than normal RO, to produce a permeate.
Electrochemical Treatment of Non-traditional Water Sources
We are interested in combining electrochemistry, materials R&D, and computation/modeling to assist in creating materials and technologies that are industrially viable for low-cost water treatment of non-traditional water sources. Selective ion mining from underutilized water sources, and reductive based chemical transformations are areas of emphasis, with the ultimate goal being to reduce the energy intensity and cost of water treatment while enabling valuable product recovery from otherwise untapped water sources.
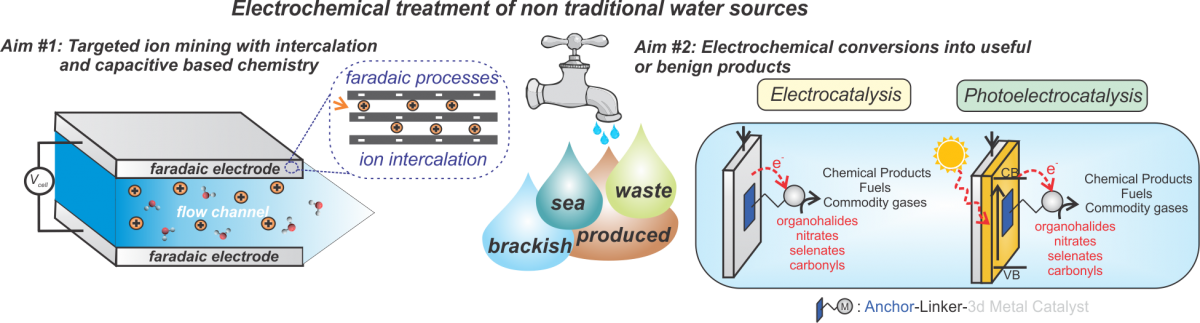
In particular, we are studying desalination using intercalation host compounds, which are examples of redox-active materials that undergo faradaic reactions and have the potential for higher salt adsorption, lower energy consumption, and tunable ion selectivity. For cation intercalation, an electrode material under an applied voltage bias undergoes a faradaic electron transfer resulting a more negative charge (redox atoms in the crystal are reduced). To maintain electroneutrality, cations intercalate, or are reversibly inserted, into the pores of the redox-active host crystal structure, thus desalinating the water outside the particles.
Another focus of our work is on catalytic reductive transformations via electro- or photo-catalysis which are emerging water treatment solutions. Immobilization of molecular photo or electrocatalysts on conductive materials denotes a promising avenue to couple renewable energy with water treatment for either in situ analytical sensing of water contaminants, or selective destruction of emerging organic pollutants.
Spectroscopic Probing of Phase Transitions in Ionic Liquids
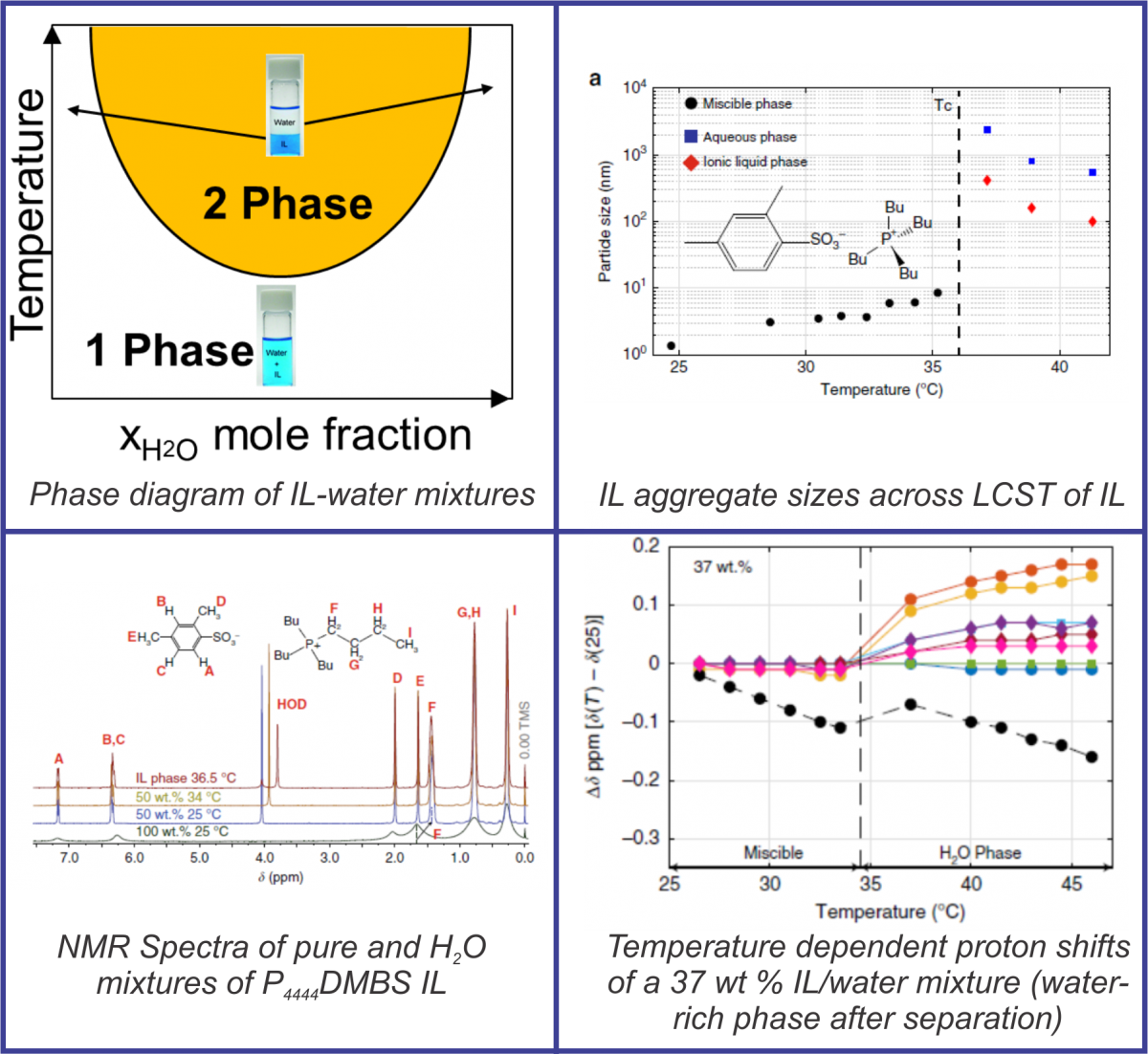
Ionic liquids (IL) that undergo liquid-liquid phase transitions past lower critical solution temperatures (LCST) with water are promising materials for water treatment. Upon heating to moderate temperatures, these solutions separate into aqueous and IL rich phases. This process happens on a fast timescale, on the order of pico- and femtoseconds. We are interested in studying these intricate phase separation mechanisms using ultrafast spectroscopic methods for deconvolution of the kinetic, thermodynamic, and structural details.
Initial fundamental studies showed that the IL forms loosely held aggregate structures which grow until their LCST, beyond which aggregate size changes significantly owing to phase separation. Multidimensional Nuclear Magnetic Resonance (NMR) study provided insight on the ILs molecular structure and its changes with temperature; this is a critical contributor to LCST phase transformations.